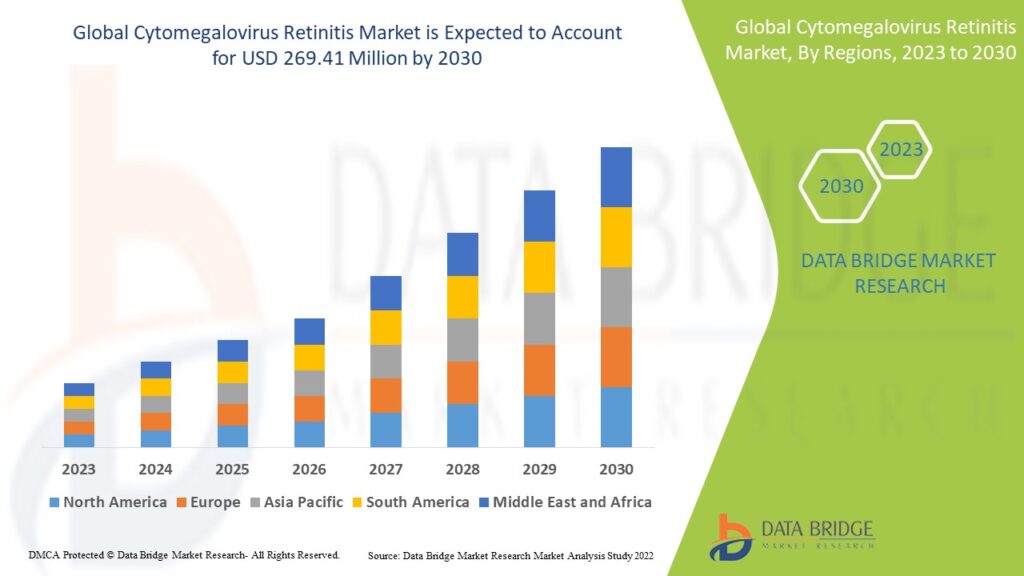
The global cytomegalovirus retinitis market size was valued at USD 183.97 million in 2023 and is projected to reach USD 284.49 million by 2031, with a CAGR of 5.60% during the forecast period of 2024 to 2031.
Cytomegalovirus (CMV) retinitis market remains a critical concern in ophthalmology, primarily affecting immunocompromised individuals such as those with HIV/AIDS, organ transplant recipients, or patients undergoing chemotherapy. If left untreated, this viral eye infection can rapidly progress to irreversible vision loss, making early detection and aggressive treatment essential.
While cases have declined in regions with widespread antiretroviral therapy, CMV retinitis continues to pose challenges—especially in low- and middle-income countries that face barriers to early diagnosis and comprehensive care. Against this backdrop, the global market for CMV retinitis treatment is evolving dynamically, shaped by innovation in antiviral therapies, diagnostic advancements, and shifting healthcare infrastructure landscapes.
Cytomegalovirus Retinitis Market Overview and Growth Drivers
The Cytomegalovirus Retinitis Market extends far beyond the core antivirals traditionally used for this infection. Its broad spectrum includes:
- Injectable antivirals such as ganciclovir, foscarnet, and cidofovir
- Oral prodrugs like valganciclovir
- Intravitreal implants and injections targeting localized delivery
- Off-label and innovative treatments such as adoptive T‑cell therapy and newer antiviral agents
Key forces propelling the market forward include:
- Persistent immune suppression—with HIV/AIDS, transplant therapy, and cancer treatments still prevalent across global populations
- Off-label and combination treatments gaining traction for enhanced efficacy and reduced resistance
- Expansion of medical infrastructure and screening initiatives, particularly in regions like India and China
- Rising awareness among patients and healthcare providers about the urgency of early intervention
- New frontiers in therapy, including novel antivirals and immune-based options
Regional Cytomegalovirus Retinitis Market
Dynamics North America & Europe
Well-established healthcare systems, strong monitoring and screening programs, and widespread antiretroviral availability contribute to robust market penetration—though increasing treatment costs and the shift toward outpatient care are reshaping delivery models.
Asia-Pacific
This region is seeing the most rapid market growth. Countries like India and China are witnessing rising investments in healthcare, increased transplant rates, and expanded early screening efforts. These developments are complemented by strategic initiatives to improve disease awareness and healthcare access in rural communities.
Latin America & Middle East/Africa
Growth here is steady, though hampered by healthcare budget limitations and lower awareness. Nevertheless, pilot programs in major urban centers and NGO-led vision health campaigns are beginning to build momentum.
Treatment Modalities in Focus
1. Systemic & Intravenous Antivirals
Medications like ganciclovir, foscarnet, and cidofovir deliver widespread antiviral coverage—but require inpatient monitoring due to significant side effects and toxicity risks.
2. Oral Medications
Valganciclovir’s bioavailability offers convenience and outpatient treatment possibilities; it continues to gain preference for less severe and peripheral CMV retinitis cases.
3. Intravitreal Therapy
Local administration via implants or injections allows high drug concentration exactly where it’s needed, minimizing systemic side effects. However, it necessitates repeat procedures every several months.
4. Off‑Label & Emerging Approaches
With concerns around drug resistance and safety, off‑label antiviral use has risen. Additionally, advancements in T‑cell therapy and novel oral agents provide encouraging alternatives, particularly for treatment-resistant or transplant-associated CMV cases.
Market Challenges
Despite promising growth, the market faces headwinds:
- High cost and toxicity of standard antiviral regimens
- Limited access to diagnostics and specialists in underserved regions
- Absence of universal treatment guidelines, leading to inconsistent care and off‑label use
- Drug resistance, demanding new combination therapies and tighter monitoring
- Shrinking case numbers in regions with effective antiretroviral usage, reducing commercial incentives
Future Growth Opportunities
1. Expanding Early Detection
Deploying retinal screenings and fundus exams in HIV clinics, transplant units, and oncology centers can allow for timely intervention before vision loss occurs.
2. Decentralizing Treatment Access
Oral antivirals and local therapies enable care delivery in clinics and outpatient settings, reducing dependence on hospital infrastructure.
3. Advancing Novel Therapies
Promising treatments like adoptive T-cell therapy and new antivirals with improved safety profiles can address resistant or recurrent cases—particularly in transplant patients.
4. Enhancing Healthcare Infrastructure
Investments in public health labs, local pharmaceutical production, and provider training can significantly strengthen CMV retinitis care frameworks in high-burden regions.
5. Fostering Collaboration Across Sectors
Partnerships between governments, NGOs, pharmaceutical companies, and healthcare institutions can help standardize care, drive awareness, and facilitate research into next-gen diagnostics and therapies.
Strategic Insights for Stakeholders
- Pharma & Biotech Companies
Focus on novel oral antivirals, extended-release intravitreal options, and immune-based therapies to meet evolving demands. - Healthcare Providers & Policymakers
Invest in robust screening programs, especially in high-risk populations; adopt treatment protocols to ensure early and standardized care. - Charitable Organizations & NGOs
Launch targeted awareness campaigns in rural or underserved areas to educate at-risk individuals and improve access to eye care. - Investors & Market Analysts
Monitor the scalability of new therapy platforms and funding trends in emerging markets, where both volume and pricing opportunities exist.
Conclusion
The global market for cytomegalovirus retinitis treatment is at a crossroads: advancing antiviral technologies and healthcare capabilities are intersecting with ongoing barriers like access and affordability. As the world pivots toward early detection, decentralized care, and innovative therapies, there’s a tremendous opportunity to reshape CMV retinitis from a blinding disease into a manageable condition—especially in regions traditionally overlooked.
Success will require a multi-pronged approach: streamlined diagnostics, safer and more accessible treatments, updated care protocols, and robust health system partnerships. Stakeholders across the spectrum—pharma, providers, governments, and NGOs—have a definitive opportunity: saving and restoring sight for thousands of vulnerable individuals worldwide. This is not just a market opportunity—it’s a mission with profound human impact.
Browse More Report: https://www.databridgemarketresearch.com/reports/global-cytomegalovirus-retinitis-market


